The EU General Product Safety Regulation (GPSR) is the 2024 law covering all consumer products marketed and/or sold to the EU market. One of the requirements of this law is that economic operators perform a risk analysis for all products placed for sale on the market. Many of our members have asked how to conduct a GPSR risk analysis for baby carriers, and this guide should assist in both conducting the risk analysis and in creating the required technical documentation.
Please note that although we refer to the EU throughout this article, there are countries outside the European Union who have or will adopt the GPSR in whole or part, and the details in this article apply to those places as well.
To read the regulation in its entirety, you can view it on the European Union’s official website. https://eur-lex.europa.eu/EN/legal-content/summary/general-product-safety-regulation-2023.html
Resources to have on hand before completing a risk analysis for baby carriers
It may be helpful to attend KidSafe’s free online Kid Safety Design Toolkit before conducting your risk analysis, as they broadly cover how to assess for and mitigate product risk through each step in the product manufacturing lifecycle.
Health Canada and the United States Consumer Product Safety Commission have issued a joint document called “Guidance on the Application of Human Factors to Consumer Products” that describes in detail how to account for real-life use for consumer products such as baby carriers.
The Risk Assessment for Consumer Products Annex from the ISO 10377:2013 standard offers evidence-based guidance, tips, and a flowchart to aid you in conducting a risk analysis for baby carriers or other products.
The EU General Risk Assessment Methodology and the Commission Implementing Decision (EU) 2019/417 and its directive on conducting a risk analysis both offer excellent guidance for conducting risk analyses, including charts and examples, although these documents are complex. Much of the information below has been taken from these two documents.
Who must conduct a risk analysis according to the GPSR?
If you are a babywearing manufacturer OR if your brand engages in private labeling, and if you market or sell your products to people in the EU (or related markets), you should conduct a risk analysis for your baby carriers.
There is no exemption for micro-enterprises, so this requirement will apply to you regardless of how small your business is.
This includes:
- manufacturers of “conversions,” where wraps or other carriers are redesigned or repaired
- carriers created to be given away for free as a charitable enterprise
- carriers made outside the EU but marketed for direct purchase by EU consumers
- hip seats, soft carriers, slings, wraps, accessories, and any other carrier or carrier-adjacent product or accessory even if it does not fall under the scope of another standard or technical report
In sum, EVERY product sold to consumers in the EU market requires a risk analysis. This includes products sold into the market via distance sales. If you manufacture, import, or private label any products with the intent to sell to the European Union (including marketing to the EU even if you sell direct-to-consumer in another country), you are bound by the GPSR.
What information must I include in a risk analysis of my baby carrier product?
The EU has laid out a basic template skeleton for documenting risk assessments for baby carriers and other products. There is no required format, but at the very least, you must include this basic information in the technical documentation of your risk analysis.
Product identification:
- brand
- name of product
- model type/batch/serial number/other identification element
- product description
- picture of product
- packaging description
- picture of packaging (we suggest including all information included in the packaging, such as instructions)
Characteristics and composition of product:
- characteristics
- material
- composition
Risk analysis and risk mitigation measures:
- Potential risks (repeat for each identified potential risk)
- Description of potential risk (ie “fall hazard”)
- Measures to address this potential risk
- All substances used in the product and packaging comply with ….
- The …. complies with European standard ….
- Warnings and instructions for use are provided ….

What are the steps to conducting a risk analysis?
There is no single “right” way to conduct a risk analysis, but the European Commission has laid out multiple detailed documents outlining guidance for risk analyses. These may be overwhelming to read and follow, so the BCIA is finalizing a training for members to assist our members in utilizing this framework to evaluate baby carriers.
The Commission Implementing Decision (EU) 2019/417 and its directive on conducting a risk analysis was developed to provide guidance for the predecessor of the GPSR (called the GPSD). Specifically, the “risk assessment guidelines … [are] an integral part of the RAPEX Guidelines. They are the instruments that enable determining the level of risk of a product and therefore help to identify the measures to be adopted.” It was written primarily with an eye to identifying whether products should be recalled, but the methodology gives clear guidance to companies conducting their GPSR risk analysis for baby carriers or other consumer products.
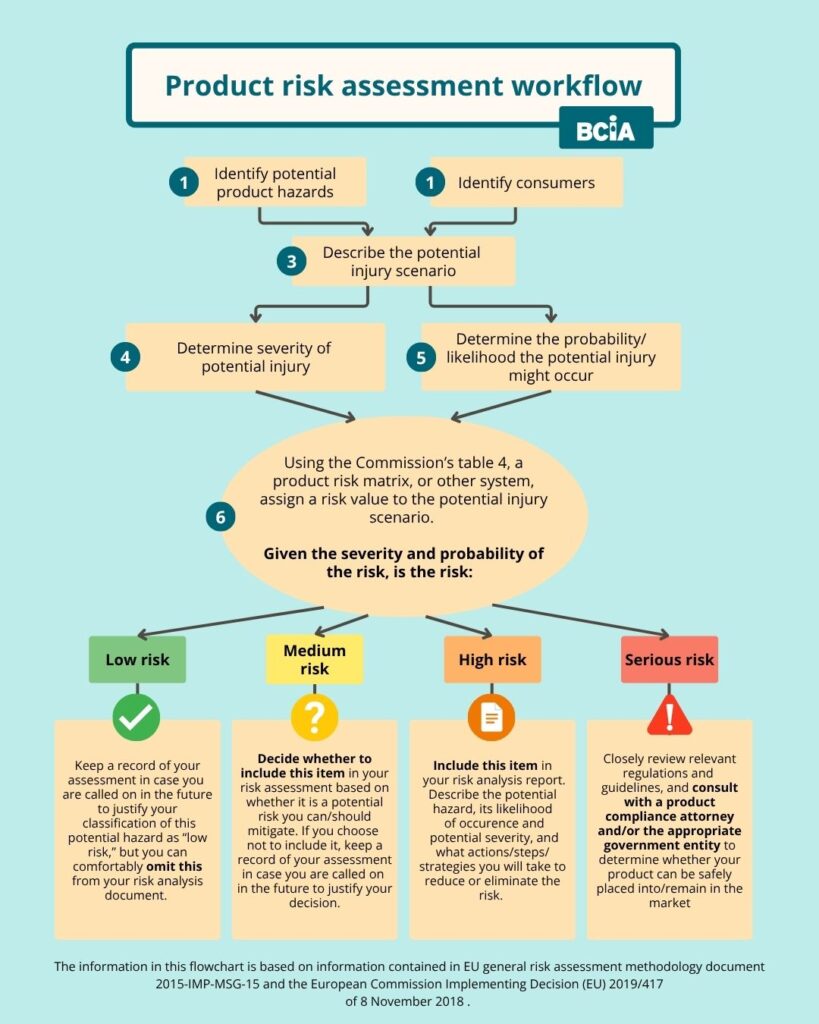
1. Identify potential GPSR risks for your baby carriers
Describe the product and its potential hazards unambiguously. When doing this, consider not only the intended use but also potential misuse such as incorrect assembly, unintended interactions, or use by unintended user groups. You should also consider product appearance, including risks related to food-imitating products that children might accidentally ingest, digital safety for connected or smart products, and interoperability (does the product present new risks when used alongside common accessories or third-party items?).
To identify potential risks, you can look at a variety of resources. Any national or international standards related to your product will likely suggest safety warnings for the most important identified risks. There is also a great deal of data available, such as:
- product recalls listed on platforms such as SafetyGate, SaferProducts, and Health Canada’s website
- data collected by ASTM (available to ASTM members)
- data collected by government organizations
- annual overview of incidents and safety data available to BCIA members on this website
- customer feedback
- real-life product testing conducted by your organization
When evaluating product risks, you should also consider human factors. The Canadian and United States governments partnered to create an excellent guide called “Application of Human Factors to Consumer Products.”
You can also consult with an experienced babywearing educator, especially one who has worked in product development. The BCIA can point you in the direction of BCIA members who work in this capacity.
2. Identify the consumers who may use your products and/or be impacted by the product risks you’ve identified
When you are considering consumers who may use or be impacted by your products, consider parents, caregivers, people the product may be handed down to, and bystanders who might be impacted.
In general, there will be little risk to bystanders from baby carriers – this generally refers to issues such as nearby people who might be struck by debris from a chainsaw, for instance. However, it is good to consider older children who might interact with the baby carrier and could access the waist buckle or other components not accessible to the child being actively carried in the product.
3. Describe the injury scenario
Consider the steps that would lead up to potential injury. This should be both detailed and concise, considering the “shortest path to injury.”
4. Assess each potential injury or risk for severity
For each risk, you need to assign it a value based on a combination of two factors: the severity of potential injury and the likelihood the risk will occur.
5. Assess each potential injury or risk for probability of occurrence
How likely is the identified risk or injury to occur?
6. Evaluate the combined severity and likelihood of injury, and assign it a risk value.
This is a daunting task, as it can be difficult to quantify injuries. If a risk is severe–such as the risk of suffocation –but the risk is almost negligible– affecting fewer than one child per year–how do you compare that to a risk of adverse adult posture in a carrier, which is far less serious but occurs much more frequently?
There are many tools that organizations use to quantify severity of potential injuries. You can utilize the tools created by others, or you can create your own system.
The Commission Implementing Decision (EU) 2019/417 and its directive on conducting a risk analysis also has examples of how they would quantify such risks, and there is another example in the EU General Risk Assessment Methodology. You can also search the web for “product risk analysis matrix” and find several examples of other matrices that might assist you in this portion of the analysis.
7. Document your plan for addressing each identified risk
Use the flow chart to see which levels of risk should be documented in your technical report. The risk levels are based on the tables and guidance in the official European Commission documents linked within this article. If you have used a different system, your classifications may differ from those in our flow chart.
Next, determine whether the risk can be reasonably addressed by product design or literature.
For each risk that can be addressed or reduced, describe how you will address, reduce, remove, or minimize the risk. This may include actions such as redesigning the product, creating or improving safety instructions and marketing, updating your product warnings, or considering your marketing practices.
What technical documentation is required with my risk analysis for baby carriers or other consumer products, and how long must I store it?
Generally, a complete technical file should include at least the following:
- The risk analysis documentation, in any format, provided it meets the requirements detailed above
- Labels and, if applicable, Instructions for Use (IFUs)
- Photographs of the product and its components
- Copies of test reports
All technical documentation must be retained and made available to market surveillance authorities for no less than 10 years.
Additionally, the person responsible for product placed into the EU marketplace must regularly evaluate products to ensure they still comply with all required technical documentation and other requirements.
Do you need more help or information?
The BCIA provides extensive support and resources for our members from all over the world. This includes support complying with European government regulations such as the GPSR.
If you are a member and have questions about performing a risk analysis for baby carriers or other products you sell, please attend our monthly office hours or send an email.
And if you’re not a BCIA member yet? This is a great time to learn more about what the Baby Carrier Industry Alliance can offer your business.
